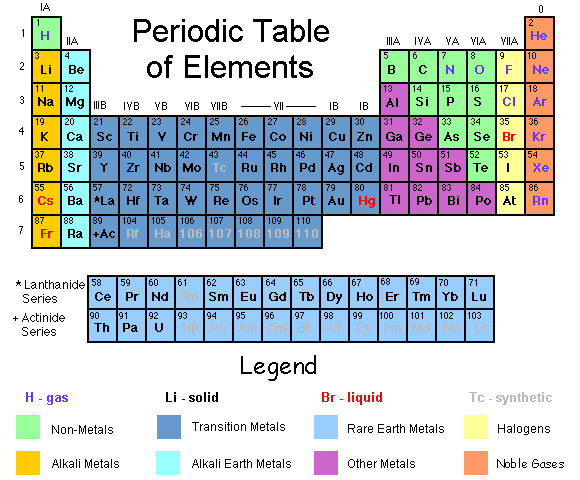
(above) A version of the modern periodic table. Not as scientific as other ones, but it makes it easy for us to grasp the general idea behind it.
The modern periodic table is sectioned off into 3 main groups. The METALS on the left, then the NON-METALS and the NOBLE GASES on the far right. Each element belongs to one of these groups because they share certain properties. The vertical columns in the table are called families. A family is a group of elements that have similar chemical properties. For example, the first column on the left are in a family called Alkali Metals. They are in this family because they share one property which is that they are very reactive due to the fact that they all have one electron in their outer orbit. The horizontal rows on the table are called periods. When going from left to right, each period goes from metal to non-metal to noble gas. The periodic table which evolved from Mendeleev's table is used in almost every science classroom in the world.